Certificates
Our production and quality system has met the GMP or cGMP standards of China, the EU, the US, Japan, and South Korea.We also passed the regulatory inspections by Chinese NMPA, EU EDQM, US FDA, Japanese PMDA, and South Korea MFDS.
2016
We passed the on-site inspection from PMDA .
2018
We passed the on-site inspection from EUEDQM.
2021
We passed the on-site registration and GMP compliance inspection for Caspofungin Acetate from CFDI and ZJMPA.
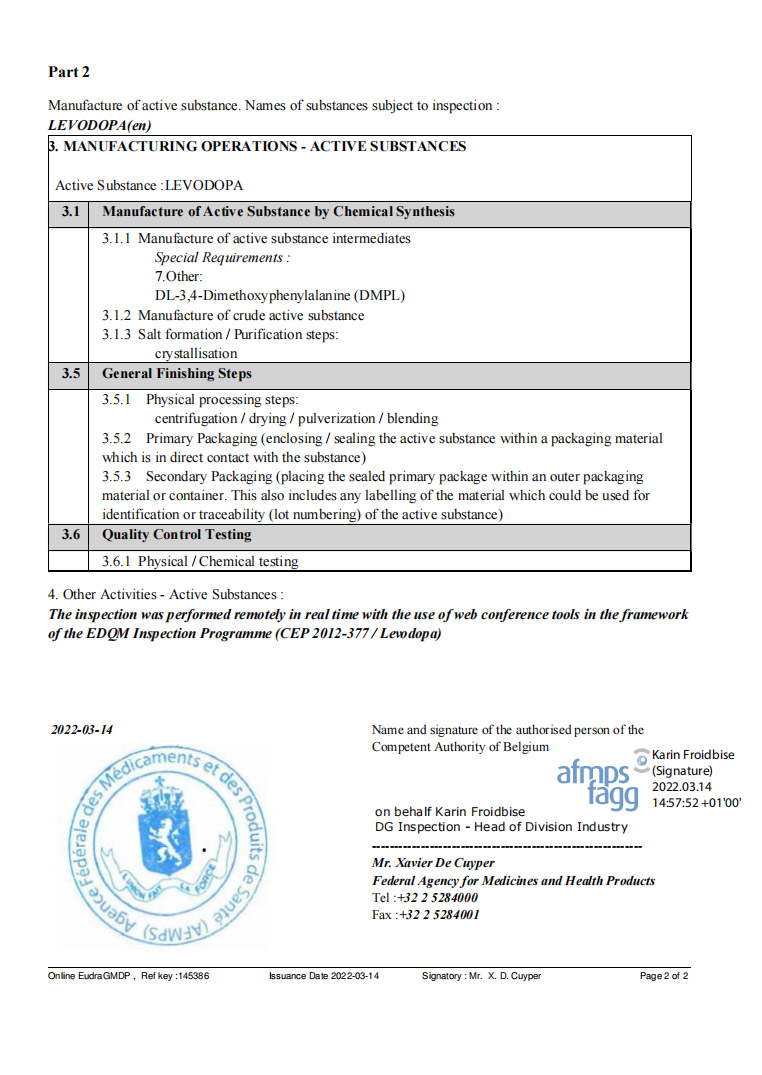
We passed the remote inspection from EU EDQM .
We passed the 4003 form document review from US FDA for Sirolimus.
2022
We passed the document review of regular GMP compliance from Japanese PMDA for Carbidopa.
2023
We passed the on-site registration and GMP compliance inspection for Caspofungin Acetate from CFDI and ZJMPA.
We passed the on-site GMP compliance inspection of South Korea MFDS for Carbidopa and Levodopa.
2024
We passed the on-site GMP compliance inspection for Levodopa from ZJMPA.
We passed the on-site pre-approval inspection from US FDA for Caspofungin Acetate.

 中文
中文





 Mobile
Mobile